Latest in Company News
Who is LightScalpel? USA-Based Designer, Manufacturer, Innovator of Exclusive Flexible Fiber Surgical CO2 Lasers
LightScalpel provides dentists and surgeons with the highest quality, state-of-the-art, precise, reliable, and affordable surgical flexible fiber carbon dioxide laser solutions. LightScalpel builds, services, distributes and sells its lasers from its facility located in Bothell, WA, USA (nearby Seattle). This video welcomes you to tour our factory and meet our people.
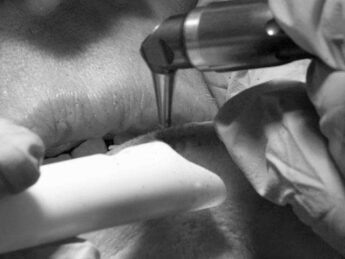
Aerosols, viruses and laser plume management for LightScalpel, VetScalpel, Aesculight and Luxar surgical CO2 lasers
ANSI Z136.3 Standard for Safe Use of Lasers in Health Care () defines laser plume as one of the non-beam laser hazards since it contains viral, bacterial, and other cellular and aerosolized particulates. ANSI Z136.3 Standard also specifies safety measures to mitigate the laser plume hazards, i.e. the mandatory use of Local Exhaust Ventilation (LEV) device equipped with a proper filter (with ANSI Z136.

LightScalpel CO2 Lasers Are Coming to Australia
LightScalpel Inc is proud to announce that this past September of 2019, LightScalpel has successfully completed the Medical Device Single Audit Program (MDSAP) recertification with the addition of Australia requirements, which is ISO’s mandatory process for LightScalpel laser products to become available outside of USA and Canada. LightScalpel is excited to soon bring its proprietary CO2 lasers with exclusive fiber delivery to patients and practitioners in Australia, where the approval from the Australian TGA is pending.
LightScalpel Earns a Spot in Dentistry Today’s Top 100 Products of 2023
We are thrilled to announce that LightScalpel has been recognized as a top 100 product of 2023 by the esteemed dental magazine, . This marks a significant milestone in our journey, reflecting our commitment to providing top-notch dental solutions.
Dentistry Today, in its 37th Annual Top 100 Products edition, has highlighted the most innovative and impactful products in the dental industry.

New Non-Ablative Laser Tissue Remodeling Handpiece from LightScalpel
a leading designer and manufacturer of surgical CO2 lasers for a variety of dental and medical applications, is in the finishing stages of releasing its Non-Ablative Laser Tissue Remodeling Handpiece for intra-oral uses, e.g. non-ablative laser soft palate treatment, etc.
We are honored to collaborate with prominent researchers, including ENT Sleep Surgeon , and Oral and Maxillofacial Surgeon , in evaluating the safety and efficacy of LightScalpel’s newest handpiece.
Luxar Laser End of Life Announcement
, as the exclusive manufacturer-authorized USA and Canada service provider for Luxar Lasers, has made every effort to continue to provide service for these obsolete models since 2008. However, this has become increasingly difficult as many critical parts and components are no longer available. With our customers' best interests in mind, we have been forced to make the difficult decision to discontinue service and repairs of the LX-20 series of Luxar Lasers.